REC-994 is being developed by Recursion Pharmaceuticals specifically for treating symptomatic cerebral cavernous malformations.
REC-994 is being developed by Recursion Pharmaceuticals specifically for treating symptomatic cerebral cavernous malformations.
The Phase I trial of REC-994, using healthy volunteers, was completed in late 2020. A Phase II trial testing the safety and tolerability of the medicine in patients with CCM enrolled its first patient in March 2022. It completed the enrollment of 60 patients ahead of schedule in June 2023. Patients were randomized into three groups: a high-dose group (400 mg/day), a low-dose group (200 mg/day), and a placebo group. This is a double-blinded trial, meaning neither the participants nor the study staff knows which group has been assigned.
What happens now?
The Phase 2 clinical trial will continue through June 2024. Patients who complete the trial before this can participate in a 12-month trial extension if they choose. Participants and the study site staff will remain blind to whether the participant has been receiving medicine or a placebo. However, participants who had been receiving a placebo will be randomly assigned to either the high-dose or low-dose medicine group. Those who were receiving medicine will continue at the same dose. It is not yet known what will happen at the end of the 12-month extension.
Please check clinicaltrials.gov to learn more.
STUDY SITES
CURRENTLY ENROLLED SITES
Arizona
Xenoscience/21st Century Neurology
Principal Investigator: Dr. Stephen Flitman
Study Contact:
(602) 274-9500
Mona Dever mdever@xenoscience.com
Meaghan Geiger mgeiger@xenoscience.com
California
Stanford University
Principal Investigator: Dr. Gary Steinberg
Study Contacts:
650-497-3968
Sasha Alexander sashalex@stanford.edu
Guiping Qui gqin68@stanford.edu
UCLA
Principal Investigator: Dr. Anthony Wang
Study Contact:
310-794-3788
Melissa Arevalo Mfarevalo@mednet.ucla.edu
Florida
University of Florida, Gainesville
Principal Investigator: Dr. Hans Shuhaiber
Study Contact:
Dr. Hans Shuhaiber (please be sure to copy Dr. Shuhaiber on your email)
hans.shuhaiber@neurology.ufl.edu
Jessica Spana Jessica.Spana@neurology.ufl.edu
Cleveland Clinic Florida, Port Saint Lucie
Principal Investigator: Dr. Marc Alain Babi
Study Contact:
Angelic Gamez 772-696-6837 GamezA3@ccf.org
Irene Ball 772-419-2146 BallI@ccf.org
Baptist Health
Principal Investigator: Dr. Ricardo Hanel
Study Contact:
Contact: Jordan Oberhaus 904-202-7089 Jordan.Oberhaus@bmcjax.com
Contact: Eisley Charltray 904-202-7998 Eisley.Charltray@bmcjax.com
Georgia
Emory University Hospital
Principal Investigator: Dr. Brian Howard
Study Contact:
Yvan Bamps, PhD ybamps@emory.edu (404-778-7673)
Backup contact:
Emilee Wehunt emorga9@emory.edu (404-778-3746)
New Jersey
Valley Hospital, Ridgewood
Principal Investigator: Dr. Dorothea Altschul
Study Contact:
201-447-8453
Kimberly Michel Kmichel@valleyhealth.com
Naomi Hatula Nhatula@Valleyhealth.com
New York
Columbia University Medical Center
Principal Investigator: Dr. Sander Connolly
Study Contact:
212-305-6071
Angela Velazquez Agv2113@cumc.columbia.edu
University of Rochester Medical Center
Principal Investigator: Dr. Michel Berg
Study Contact:
585-275-0404
Cathleen Concannon Cathleen_Concannon@urmc.rochester.edu
Pennsylvania
Penn Medicine
Principal Investigator: Dr. Jan Karl Burkhardt
Study Contact:
Leah Coghlan NCRDNeuroICU@uphs.upenn.edu
Thomas Jefferson University
Principal Investigator: Dr. Pascal Jabbour
Study Contact:
Contact: Nadirah Jones Nadirah.Jones2@jefferson.edu
University of Pittsburgh
Principal Investigator: Dr. Zenonos
Study Contact:
Sara Onesi
412-385-7711 onesisa2@upmc.edu
Texas
The University of Texas Southwestern Medical Center
Principal Investigator: Dr. Jonathan White
Study Contact:
Tash Mupambo Tashinga.Mupambo@UTSouthwestern.edu
Emerson Nairon Emerson.Nairon@UTSouthwestern.edu
Virginia
University of Virginia
Principal Investigator: Dr. Ryan Kellogg
Study Contact:
Judy Beenhakker, MS, CCRC
P: 434-982-1856
jgb3p@virginia.edu
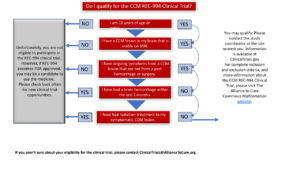
ELIGIBILITY CRITERIA FROM Clinicaltrials.gov NCT05085561
Ages Eligible for Study: 18 Years and older (Adult, Older Adult)
Sexes Eligible for Study: All
Accepts Healthy Volunteers: No
- 18 years of age or older with anatomic CCM lesions demonstrated by brain MRI
- Have symptomatic CCM
- Have provided written informed consent to participate in the study
- Have NOT participated in a clinical trial utilizing an investigational agent within 28 days or within 6 half-lives of the investigational drug (whichever is longer) prior to Screening
Exclusion Criteria:
- Symptoms deemed by the study Investigator to be caused exclusively by irreversible neuronal damage from prior stroke or neurosurgical instrumentation
- History of cranial irradiation or surgical/radio-surgical treatment of the primary symptomatic CCM lesion
- Pregnant or breastfeeding
- Unable or unwilling to participate in MRI assessments (eg, claustrophobia, metal implant, implanted cardiac pacemaker). This trial does not use contrast.
- Liver dysfunction or active liver disease as defined by baseline serum transaminases >2x upper limit of normal (ULN)
- Have severely impaired renal function (eGFR <60ml/min) or active renal disease
- Have had a previous diagnosis of skeletal muscle disorders (myopathy) of any cause or have a baseline creatine kinase level > 5x ULN
- History of alcohol or substance abuse within 1 year prior to screening
- Clinically significant laboratory abnormality
- Have had an intracerebral hemorrhage within 3 months of screening or any brain surgery within 6 months of screening
More background
REC-994 is the first industry-sponsored trial of a medicine developed specifically for CCM. The following webinar offers more information about its unique history.
Join one of our patients as he begins participating in the clinical trial.
READ MORE
In this blog post, Recursion’s CEO Chris Gibson shares the origin story of REC-994 beginning in graduate school at the University of Utah.
Updated 06.14.23